
INTRODUCTION
Primary DLBCL of the CNS (PCNSL) is a rare, extra-nodal non-Hodgkin lymphoma with high risk of relapse without consolidation therapies. AutoHCT has emerged as an accepted consolidation strategy, especially with increasing use of CNS penetrant chemotherapy agents in transplant conditioning. However, studies defining the optimal conditioning regimen for PCNSL have not been performed. The use of two common conditioning regimens in the U.S., thiotepa/busulfan/cyclophosphamide (TBC) and thiotepa/carmustine (TT-BCNU), is supported by phase II trials, but lack comparative data. Extrapolating experience from systemic lymphomas, BEAM (BCNU/etoposide/cytarabine/melphalan) is also prescribed for PCNSL in the U.S. Using the Center for International Blood & Marrow Transplant Research (CIBMTR) registry, we compared outcomes for PCNSL patients undergoing autoHCT using the three most commonly employed conditioning regimens: TBC vs. TT-BCNU vs. BEAM.
METHODS
Six hundred and eight adult PCNSL patients who underwent a first autoHCT between 2010-2018 were included. Conditioning regimens included TBC (n = 265), TT-BCNU (n = 278), and BEAM (n = 65). The primary endpoint was progression-free survival (PFS). Secondary endpoints included (a) time to hematopoietic recovery, (b) relapse/progression, (c) non-relapse mortality (NRM), and (d) overall survival (OS). Kaplan-Meier method was used to estimate probabilities of PFS and OS. Cox proportional hazard analysis was used to identify prognostic factors for relapse, NRM, PFS, and OS. Variables considered in regression model include patient age, sex, race, performance status, HCT-comorbidity index (HCT-CI), rituximab use during conditioning, interval between diagnosis and HCT, and remission status. Covariates with a p<0.05 were considered significant.
RESULTS
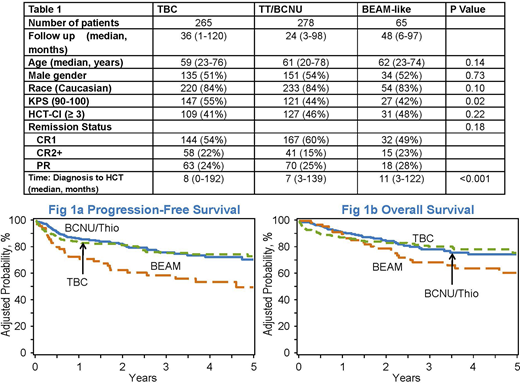
Baseline characteristics are presented in Table 1. The groups were comparable with regards to median patient age, gender, race, HCT-CI, and remission status. The cumulative incidences of neutrophil recovery at 1-month for the three conditioning regimens were: TBC 96% (95%CI 94-98%), TT-BCNU 100% (95%CI 98-100%), and BEAM 100% (95%CI 100%), (p < 0.001). The cumulative incidence rate of relapse for TBC, TT-BCNU, and BEAM at 1-year were 6% (95%CI 3-9%), 10% (95%CI 7-14%), and 23% (95%CI 14-35%), respectively (p = 0.003). The corresponding cumulative incidence rate of relapse at 3-years were 11% (95%CI 7-16%), 15% (95%CI 10-20%) and 37% (95%CI 25-50%) (p < 0.001) respectively. The adjusted NRM were higher for TBC: 100-day 7% (95%CI 4-10%) and 1-year 11% (95%CI 7-15%), compared to TT-BCNU: 100-day 2% (95%CI 0.2-3%) and 1-year 4% (95%CI 2-6%), and BEAM: 100-day 0% (95%CI 0%) and 1-year 4% (95%CI 0-9%) (1-year p=0.01). The 3-year adjusted PFS across the three conditioning regimens were: TBC 75% (95%CI 69-81%), TT-BCNU 76% (95%CI 70-82%), and BEAM 58% (95%CI 46-70%) (p = 0.03) [Figure 1a]. The adjusted OS at 3-years were: TBC 81% (95%CI 75-86%), TT-BCNU 78% (95%CI 72-85%) and BEAM 69% (95%CI 58-80%), (p = 0.17) [Figure 1b]. Relapse of primary disease was the most common cause of death in all three cohorts: TBC 38% (n=20), TT-BCNU 72% (n=33) and BEAM 76% (n=19). Other significant causes of death in the TBC group included infections 15% (n=8) and organ failure 21% (n=11).
CONCLUSIONS
In this CIBMTR analysis in patients with PCNSL, we found favorable outcomes with thiotepa-containing conditioning regimens. Adjusted 3-year PFS favored TBC and TT-BCNU over BEAM and suggest that use of BEAM should be discouraged in this specific setting. Whether TBC or TT-BCNU is the optimal conditioning regimen requires further inquiry in a prospective clinical trial.
Scordo:McKinsey & Company: Consultancy; Omeros Corporation: Consultancy; Kite - A Gilead Company: Other: Ad-hoc advisory board; Angiocrine Bioscience, Inc.: Consultancy, Research Funding. Kharfan-Dabaja:Pharmacyclics: Consultancy; Daiichi Sankyo: Consultancy. Herrera:Pharmacyclics: Research Funding; Karyopharm: Consultancy; Bristol Myers Squibb: Consultancy, Other: Travel, Accomodations, Expenses, Research Funding; Merck: Consultancy, Research Funding; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding; AstraZeneca: Research Funding; Immune Design: Research Funding; Seattle Genetics: Consultancy, Research Funding. Hamadani:Janssen R&D; Incyte Corporation; ADC Therapeutics; Celgene Corporation; Pharmacyclics, Omeros, AbGenomics, Verastem, TeneoBio: Consultancy; Sanofi Genzyme, AstraZeneca: Speakers Bureau; Takeda Pharmaceutical Company; Spectrum Pharmaceuticals; Astellas Pharma: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees. Sauter:Sanofi-Genzyme: Consultancy, Research Funding; Spectrum Pharamaceuticals: Consultancy; Novartis: Consultancy; Genmab: Consultancy; Precision Biosciences: Consultancy, Research Funding; Kite - a Gilead Company: Consultancy; Celgene: Consultancy, Research Funding; Gamida Cell: Consultancy; GSK: Consultancy; Bristol-Myers Squibb: Research Funding; Juno Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal